Get a used textbook. Chemistry is great! |
|
Results 1 to 25 of 26
Thread: Tell me about HS Chemistry
-
02-24-2011 07:09 AM #1
Tell me about HS Chemistry
See title above, I'm not Chemistry, but it's something of interest and I'd like to get myself involved in now to be ahead later on.
So does anyone have any links or key information I should start reading up first?
~Thanks in advance for any responses"Over thinking, over analyzing separates the body from the mind."
-
02-24-2011 08:00 AM #2
-
02-24-2011 11:06 AM #3
I remember a bit from high school Chemistry. You might already know what I'm going to say, because it is very basic stuff and is often known as common knowledge. Just some basic concepts that the foundations are built upon:
moles:
A mol is a number which represents 6.02*(10^23) of something(^ means 'to the power of'), just like a dozen represents 12 of something. It is used while dealing with very large quantities of things like molecules, because it's easier to say there are 3.5 moles of hydrogen atoms than to say there are however many billion of them. (Just as it sounds better to say 4 dozen than 48).
atoms - very basic:
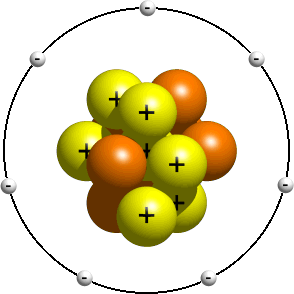
Atoms are made up of three basic particles: protons, neutrons, and electrons. Protons and neutrons are approximately the same size and mass, and together make up the nucleus of an atom, clustered in the atom's center. Electrons are extremely tiny in comparison, and fly in orbits around the nucleus. Electrons are practically massless. Electrons are said to have a negative charge, and protons are said to have a positive charge, while neutrons, as the name implies, have no charge at all. Protons and electrons have equal but opposite charges, called elementary charge.
Charge of proton: +1.602*(10^-19) C
Charge of electron: -1.602*(10^-19) C
In high school courses, you do not have to worry about memorizing the number, because after using it so much (like with the mol) it will be stuck in your head whether you willed it to be or not.
In a neutrally charged atom, there are the same number of electrons as there are protons. If an atom has 6 protons, it will also have 6 electrons.
The only difference between different atoms is the number of protons, neutrons, and electrons.
An atom is named according to its number of protons. For example, if an atom has 1 proton, it is called hydrogen. That is how a hydrogen atom is defined. Similarly, if an atom has 2 protons, it is called helium. If an atom has 8 protons, it is called oxygen, etc. Atoms are given abbreviations (H = hydrogen, He = helium, C = carbon, Ni = nickel, etc.).
In a neutral state, atoms have the same number of protons as electrons. If the numbers are unequal, though, the atoms is called charged. If an atom has 9 protons but only 8 electrons, it is missing an electron. Since an electron is negatively charged, and the atom is missing that negative charge, it is called positively charged. Similarly, if an atom has 9 protons and 10 electrons, it is called negatively charged.
Others can correct me if I've been wrong at all. I'm very tired. I might post more of the little I remember some other time.Last edited by Dianeva; 02-24-2011 at 11:13 AM.
-
02-24-2011 07:06 PM #4Banned


- Join Date
- Aug 2005
- Posts
- 9,984
- Likes
- 3084
The basics of chemistry is pretty fascinating - basically the bit that overlaps with physics - but after that it gets very dull.
-
02-24-2011 10:03 PM #5
Even if this information isn't new to you, it's good for me because I've refreshed my memory. I think I'd have been on the verge of forgetting it otherwise.

Electrons travel around the nucleus at different energy levels, which are sort of like the orbits of planets. You can think of them as invisible cocentric spheres, called electron shells. Electrons have to travel along the surface of one of these invisible spheres. There is a rule which says only 2 electrons are allowed in the first sphere, 8 are allowed in the second sphere, etc.
The electron shells fill up with electrons from closest to farthest from the nucleus. In Hydrogen, for instance, which only has 1 proton, 1 neutron, and 1 electron, the one electron resides in the lowest energy level. If the hydrogen atom gains a second electron, it will also go in the lowest energy level. So you have two electrons in that same energy level. Now, if for some reason it gained a third electron, it cannot go in the lowest energy level, because only 2 electrons are allowed to be there. It has to go up to the second energy level. So in an atom with 3 electrons, there are 2 occupying the lowest energy level, and 1 occupying the second. 7 more electrons can be added to the second energy level until it reaches its maximum of 8 electrons. The atom Neon has 10 electrons; 2 in the first energy level, and 8 in the second. If another electron is added, it is forced to go into the third energy level because the second has reached its maximum of 8.
The electrons in the highest occupied layer are called valence electrons. They get a special name because the number of electrons in the highest layer turns out to be important in determining how an atom is likely to react with other atoms. Atoms 'like' to have their orbits completely filled. A Helium (He) atom has only 1 layer of electrons, the first one, and that layer is completely filled with its 2 electrons, so Helium is 'happy' in its normal state. Similarly, Neon (Ne) has 8 valence electrons. Because its topmost layer is completely filled to its maximum capacity, Neon is also a 'happy' atom in its natural state. These atoms which naturally have filled valence shells are called noble gases.

Consider this case. A Sodium (Na) atom has 11 electrons in total: that's 2 electrons in its first shell, 8 electrons in its second shell, and 1 electron in its third (valence) shell. Na is not 'happy' with its 11 electrons. A Chlorine (Cl) atom has 17 electrons: that's 2 electrons in its first shell, 8 in its second shell, and 7 in its third shell (the maximum capacity of the third shell is also 8). Cl is also not 'happy' because its outer shell has 7 electrons instead of 8. What happens when these two atoms meet? Na has an extra electron it wants to get rid of, while Cl would like another electron. When the two atoms meet, the Na atom gives its extra electron to the Cl atom. This way, both atoms have their valence shells filled completely, and are 'happy'. Now, after this, the Cl atom has gained an electron and so is negatively charged, while the Na atom has lost an electron and so is positively charged. Opposite charges attract, so the two atoms are now attracted to one another and form a Sodium Chloride molecule. This type of bond, where one atom gives one or more electrons to another atom, is known as an ionic bond.Last edited by Dianeva; 02-24-2011 at 10:09 PM.
-
02-24-2011 10:37 PM #6Banned


- Join Date
- Aug 2005
- Posts
- 9,984
- Likes
- 3084
The truth: the above is a good model for understanding most of basic chemistry but in reality electrons aren't remotely like planets orbiting the sun. Electrons move so fast that basically one moment they'll be in one place and the next they'll be in a totally different place (although it is more likely to be in a certain place). Therefore a single electron effectively forms a 'cloud' around the nucleus, a cloud of dispersed negative charge, which is densest around where it is traditionally seen as having an orbit.
-
02-24-2011 10:38 PM #7
I'm aware of that, but as you said, it's good for understanding basic chemistry.
-
02-24-2011 10:49 PM #8
Chemistry is my second worst subject, next to maths

BUT I do think that if you're interested, you should go ahead and learn it. It's a little more complex compared to physics and biology, but then again I find them a lot easier.
Today I learned how nitric acid is made, it was quite interesting to be honest! I never thought I'd say that about ANYTHING related to chemistry.
After a good six months, I've finally learned the difference between ionic and covalent bonding. Kind of... YAY! We got a university lecturer in to teach S3 and S4 the basics of bonding. I knew most of it already but now it's just clicked

-
02-24-2011 11:21 PM #9Banned


- Join Date
- Aug 2005
- Posts
- 9,984
- Likes
- 3084
Ionic is where atom A gives atom B some electrons, so atom A becomes positive and atom B becomes negative, so atom A is attracted to atom B because they're opposites.
Covalent is where atom C and atom D both put some electrons in the gap between them, so they both become positive and the gap becomes negative, so they're both attracted to the gap.
Simples.
-
02-24-2011 11:30 PM #10
Most of high school chemistry is about stoichiometry and measuring chemical reactions (mass, energy, pH, etc.) which is very tedious and basically just applied math.
The structure of an atom and the periodic table is pretty interesting though.
-
02-25-2011 12:41 AM #11Banned


- Join Date
- Aug 2005
- Posts
- 9,984
- Likes
- 3084
Yeah, about two thirds of my chemistry lessons were totally pointless in the last couple of years at school. One third was really interesting stuff about orbitals and the pi bonds in benzene, but the rest was everybody else in the class saying 'derpy dur logarithm hur?' for hours when dealing with stuff like buffer pHs which I understood as soon as they were mentioned as just a trivial application of what I had learned elsewhere.
-
02-25-2011 03:37 AM #12
I love chemistry. Especially biochemistry. Organic is cool too. I could share plenty but I wouldn't know where to start.
-
02-25-2011 04:51 AM #13In my own mind



- Join Date
- Nov 2009
- LD Count
- Not enough
- Gender

- Location
- Corona, CA
- Posts
- 666
- Likes
- 41
- DJ Entries
- 25
If HS chem is anything like chem I took in college, it's easy... of course the teacher I had sucked. I love science and had always gotten A's, then I get a C in chem... unpossible! lol
As I said, I love science, so I loved chem. Sometimes the math was a little weird, but is applied so makes more sence than just staring at a formula
Actually, if you'd like to get into quantum physics, a single electron can be in two places at one. How fun!
But yes, he's correct on the movement of the electronsDreams Recalled since 10-31-09: 776
Best Recall in One Night: 8 (12-25-10).
DILDs: 8 (2-26-11); MILDs: 4 (7-28-10)
Goal: Play Calvinball [ ]

-
02-25-2011 06:55 AM #14Member

- Join Date
- Feb 2004
- Posts
- 5,165
- Likes
- 711
I don't think you need to know the elementary charge of stuff in high school, I don't even recall it being mentioned. Knowing that a proton has a charge of 1 and an electron has a charge of negative 1 seems sufficient. It makes me curious about what you were doing in high school that you used it all the time, and so often you memorized it.
-
02-25-2011 08:22 AM #15
-
02-25-2011 03:48 PM #16
I'm surprised no one has mentioned balancing equations yet. That is pretty standard HS chem.
-
02-25-2011 08:17 PM #17
-
02-25-2011 08:25 PM #18
Stoichiometry...
-
02-25-2011 11:11 PM #19
I actually quite like balancing equations. It's kinda like algebra I guess, which is something I can actually do when it comes to maths. I'm not really bad at maths, I'm just not good at it either. Or do better than anything else anyway.
My chemistry teacher sucks. She just kinda stumbles her way through topics, and when I ask her something she goes "We went over this in the last topic!" and I look up my notes and can't find anything to do with it. Luckily I have a friend who loves chemistry and helps me with it.
I find biology so much more interesting. Especially human biology, because it's something that's happening to you and I like learning about how we work
Physics I'm not so keen on. I find it the most boring of the sciences, but I understand it better than chemistry. It's mostly memorising formulas that I get stuck with. But I make little songs before tests that help me with them
I don't think I could ever do Higher chemistry. If I can't get standard grade, there's no way I'm getting higher. The funny thing is that my teacher thinks I'm good at it, and then I do shit in a test and she just says "you should be doing better than this, this isn't up to your standard." The reason for this is because for my first test, which was very easy (mainly atoms, periodic tables etc) I got two 1's (equivelent to two A's). Gah! I now go between 2/3/4 (B/C/D). 7 is a fail.
-
02-26-2011 12:10 AM #20
School is very different in college...especially when you go through college more than once or are seeking a higher degree.
 In high school I got B's and C's in chemistry, and did even worse in physics. My second time around in college though I'm getting straight A's. Biology was always my best subject too. But now I do equally well in all the sciences (chemistry, physics, microbiology, biochemistry, genetics, organic), and I love them all. I've become a major science nerd.
In high school I got B's and C's in chemistry, and did even worse in physics. My second time around in college though I'm getting straight A's. Biology was always my best subject too. But now I do equally well in all the sciences (chemistry, physics, microbiology, biochemistry, genetics, organic), and I love them all. I've become a major science nerd.
-
02-26-2011 12:34 AM #21Banned


- Join Date
- Aug 2005
- Posts
- 9,984
- Likes
- 3084
Just been readin' up about orbitals and apparently they expressed in terms of symmetry groups... cool beans. Gotta check that out. :V
-
02-28-2011 04:40 AM #22
-
03-01-2011 12:01 AM #23In my own mind



- Join Date
- Nov 2009
- LD Count
- Not enough
- Gender

- Location
- Corona, CA
- Posts
- 666
- Likes
- 41
- DJ Entries
- 25
I had a devil of a time with balancing equations in chem, but in math I had no problem. Guess in chem I was in science mode and math didn't make sense, but when I'm in math class I'm in math mode, so no prob, haha
It was halfway through the year when in math we had to convert stuff and I went, "Wait... this is what I've done in chem! I get it now!" Still had a hard time with it in chem though Dreams Recalled since 10-31-09: 776
Dreams Recalled since 10-31-09: 776
Best Recall in One Night: 8 (12-25-10).
DILDs: 8 (2-26-11); MILDs: 4 (7-28-10)
Goal: Play Calvinball [ ]

-
03-01-2011 01:14 AM #24The traveller Achievements:






- Join Date
- Dec 2009
- Gender

- Location
- Glasgow, Scotland
- Posts
- 1,134
- Likes
- 1243
My chemistry? I'm mostly made up of water, I guess.
-
03-01-2011 01:25 AM #25
Equation balancing can be fun once you get used to it. Once you've memorized the rules, it's really just an easy puzzle. But I suppose that can be said about a lot of subjects in school. Most people's problems are not learning the rules and memorizing what you're supposed to in the first place, then it's just boring and stressful.
Similar Threads
-
Brain chemistry/supplements experiment
By Rodulf in forum General Dream DiscussionReplies: 0Last Post: 11-15-2009, 05:08 PM -
Chemistry/Physics/Biology
By False in forum Science & MathematicsReplies: 53Last Post: 04-20-2009, 02:38 AM -
Chemistry And Polyphasic Sleep
By redclay92 in forum Sleep and HealthReplies: 6Last Post: 08-29-2008, 07:15 AM -
WILDS just a brain chemistry thing?
By DrTechnical in forum Attaining LucidityReplies: 4Last Post: 03-03-2008, 10:16 PM -
Sleep, Cycles And Chemistry
By Tornado Joe in forum Sleep and HealthReplies: 0Last Post: 09-27-2006, 03:07 PM




 5Likes
5Likes LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks


 Reply With Quote
Reply With Quote





Bookmarks